Clinical Trial Packaging and Labelling Market: Smart Packaging Trends, Supply Chain Digitalization, and Regulatory Compliance Solutions
"Market Trends Shaping Executive Summary Clinical Trial Packaging and Labelling Market Size and Share
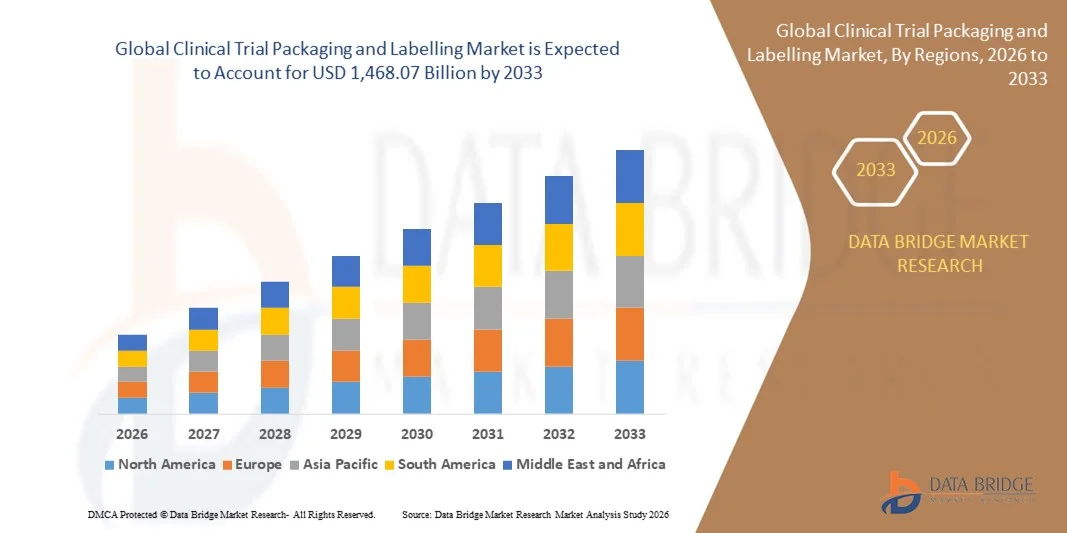
The global clinical trial packaging and labelling market size was valued at USD 854.43 billion in 2025 and is expected to reach USD 1,468.07 billion by 2033, at a CAGR of 7.00% during the forecast period
Clinical Trial Packaging and Labelling Market report performs geographical analysis for the major areas such as North America, China, Europe, Southeast Asia, Japan, and India, with respect to the production, price, revenue, and market share for top manufacturers. This market study also analyzes the market status, market share, growth rate, future trends, market drivers, opportunities and challenges, risks and entry barriers, sales channels, distributors, and Porter's Five Forces Analysis. This Clinical Trial Packaging and Labelling Market research report delivers a comprehensive analysis of the market structure along with the estimations of the various segments and sub-segments of the market.
An absolute insight and know-how of the greatest market opportunities in the relevant markets or Clinical Trial Packaging and Labelling Market industry required for successful business growth can be accomplished only with the best market research report. The Clinical Trial Packaging and Labelling report provides market potential for each geographical region based on the growth rate, macroeconomic parameters, consumer buying patterns, their preferences for particular products, and market demand and supply scenarios. All the studies performed to generate this Clinical Trial Packaging and Labelling report are based on large group sizes and also at a global level. This Clinical Trial Packaging and Labelling Market Research report provides clients with the supreme level of market data and information that is specific to their niche and their business requirements.
Unlock detailed insights into the growth path of the Clinical Trial Packaging and Labelling Market. Download full report here:
https://www.databridgemarketresearch.com/reports/global-clinical-trial-packaging-and-labelling-market
Clinical Trial Packaging and Labelling Industry Performance Overview
Segments
- On the basis of product type, the Global Clinical Trial Packaging and Labelling Market can be segmented into labels, blisters, vials & ampoules, bottles, secondary packaging, and others. Labels are crucial for providing information about the clinical trial, including dosage instructions, expiry dates, and cautionary notes. Blister packaging is commonly used to dispense medications in clinical trials, offering ease of use and protection. Vials and ampoules are used for storing liquid medications securely. Bottles are utilized for packaging larger quantities of medications. Secondary packaging includes cartons, pouches, and trays for grouping primary packaging components.
- Based on material, the market can be categorized into plastic, paper, glass, and others. Plastic packaging is widely used in clinical trials due to its durability, light weight, and cost-effectiveness. Paper packaging is eco-friendly and often used for labels and cartons. Glass is preferred for its non-reactive properties, making it suitable for storing sensitive medications.
- By end user, the market is segmented into pharmaceutical & biopharmaceutical companies, contract research organizations (CROs), research institutions, and others. Pharmaceutical and biopharmaceutical companies are the primary users of clinical trial packaging and labelling services, as they conduct a significant portion of clinical trials globally. CROs play a vital role in managing clinical trials on behalf of pharmaceutical companies and often outsource packaging and labelling tasks to specialized companies.
Market Players
- Some of the key players in the Global Clinical Trial Packaging and Labelling Market include Sharp (a part of UDG Healthcare plc), Bilcare Limited, WestRock Company, Schreiner MediPharm, Amcor plc, Thermo Fisher Scientific Inc., Klöckner Pentaplast, 3M, and CCL Industries. These companies offer a wide range of packaging and labelling solutions tailored to the unique requirements of clinical trials. With a focus on innovation, quality, and compliance with regulatory standards, these market players contribute significantly to the efficiency and safety of clinical trial processes.
- Other notable players in the market include Gerresheimer AG, Stamar Packaging, Faubel & Co. Nachf. GmbH, Origin Pharma Packaging, Parelec Inc., 3M Company, Eurofins Scientific, and WS Packaging Group, Inc. These companies bring diverse expertise and capabilities to the global clinical trial packaging and labelling sector, ensuring that pharmaceutical companies and research organizations have access to high-quality packaging solutions for their clinical trials.
The Global Clinical Trial Packaging and Labelling Market is witnessing continuous growth driven by the increasing number of clinical trials conducted worldwide. One key trend shaping the market is the rising adoption of advanced technology and automation in packaging and labelling processes for clinical trials. Automation not only enhances operational efficiency but also ensures accuracy and compliance with regulatory requirements. This trend is particularly crucial in the pharmaceutical industry, where precision and consistency in packaging and labelling are paramount to ensure patient safety and regulatory compliance.
Another significant factor influencing the market is the growing emphasis on sustainability and environmentally friendly practices. As more pharmaceutical companies and research organizations prioritize eco-friendly packaging solutions, there is a shift towards utilizing recyclable materials and reducing the overall environmental impact of clinical trials. This shift is not only driven by regulatory pressures but also by the increasing awareness among stakeholders about the importance of sustainability in healthcare practices.
Moreover, the increasing complexity of clinical trial designs and protocols is leading to a greater demand for customizable and adaptable packaging and labelling solutions. With the rise of personalized medicine and targeted therapies, the need for flexible packaging options that can accommodate diverse drug formulations and dosing regimens is becoming more pronounced. Market players are thus focusing on enhancing their product portfolios to cater to the evolving needs of the pharmaceutical industry and research institutions.
Furthermore, the COVID-19 pandemic has accelerated the digitization of clinical trial processes, including packaging and labelling activities. Remote monitoring, virtual trials, and decentralized clinical trials have become more prevalent, requiring innovative packaging and labelling solutions that support remote distribution and monitoring. This shift towards virtualization and digitalization presents opportunities for market players to develop smart packaging technologies that enable real-time tracking, temperature monitoring, and tamper-proof features.
In conclusion, the Global Clinical Trial Packaging and Labelling Market is undergoing significant transformations driven by technological advancements, sustainability concerns, customization requirements, and the impact of the COVID-19 pandemic. Market players are poised to capitalize on these trends by offering innovative solutions that address the evolving needs of pharmaceutical companies, research organizations, and other end users involved in clinical trials. By staying attuned to market dynamics and regulatory developments, companies can establish a competitive edge in this dynamic and evolving market landscape.The Global Clinical Trial Packaging and Labelling Market is poised for continued growth and evolution, driven by various factors shaping the industry landscape. One significant trend influencing the market is the increasing adoption of advanced technology and automation in packaging and labelling processes. The integration of automation not only enhances operational efficiency but also ensures precision, accuracy, and compliance with regulatory standards. This trend is particularly crucial in the pharmaceutical sector, where the safety and efficacy of clinical trial medications rely on stringent packaging and labelling protocols.
Moreover, there is a notable shift towards sustainability and eco-friendly practices within the clinical trial packaging and labelling sector. As pharmaceutical companies and research organizations prioritize environmental consciousness, there is a growing emphasis on utilizing recyclable materials and reducing the overall carbon footprint of clinical trials. This move towards sustainability is not only driven by regulatory requirements but also by a heightened awareness of the importance of eco-responsible practices in healthcare operations.
The market is also witnessing increasing complexity in clinical trial designs and protocols, leading to a demand for customizable and adaptable packaging solutions. With the emergence of personalized medicine and targeted therapies, the need for flexible packaging options that can accommodate diverse drug formulations and dosing regimens is gaining prominence. Market players are focusing on expanding their product offerings to cater to the evolving needs of the pharmaceutical industry and research institutions, thereby driving innovation and differentiation in the market.
Furthermore, the COVID-19 pandemic has accelerated the digitization of clinical trial processes, including packaging and labelling activities. The rise of remote monitoring, virtual trials, and decentralized clinical trials has underscored the importance of developing smart packaging technologies that support remote distribution and monitoring. This shift towards digitalization presents opportunities for market players to introduce solutions that enable real-time tracking, temperature monitoring, and enhanced security features, catering to the evolving landscape of virtual clinical trials.
In conclusion, the Global Clinical Trial Packaging and Labelling Market is witnessing profound transformations driven by technological advancements, sustainability initiatives, customization requirements, and the influence of the COVID-19 pandemic. The market players are well-positioned to capitalize on these trends by offering innovative solutions that meet the evolving needs of the industry stakeholders. By staying abreast of market dynamics and regulatory changes, companies can navigate the evolving market landscape and drive growth through differentiated offerings and strategic partnerships.
Check out detailed stats on company market coverage
https://www.databridgemarketresearch.com/reports/global-clinical-trial-packaging-and-labelling-market/companies
In-Depth Market Research Questions for Clinical Trial Packaging and Labelling Market Studies
- What revenue figures define the current Clinical Trial Packaging and Labelling Market?
- What are the near-term and long-term growth rates expected in Clinical Trial Packaging and Labelling Market?
- What are the dominant segments in the Clinical Trial Packaging and Labelling Market overview?
- Which companies are covered in the competitor analysis for Clinical Trial Packaging and Labelling Market?
- What countries are considered major contributors for Clinical Trial Packaging and Labelling Market?
- Who are the high-growth players in the Clinical Trial Packaging and Labelling Market?
Browse More Reports:
Global Intermittent Pneumatic Compression (IPC) Devices Market
Global Dyslexia Treatment Market
Global Ophthalmology Electronic Health Record (EHR) Market
Global Raised Garden Beds Market
Global Hot Water Dispensers Market
Global Advanced Therapy Medicinal Products Market
Global Apraxia Drug Market
Global Food and Agriculture Technology and Products Market
Global Roll-Your-Own Tobacco Product Market
Global Virus Filtration Market
Global Activity Tracking Fitness App Market
Global Thermal Inkjet (TIJ) Coder Market
Global Solenoid Valves Market
Global Anesthesia Circuits Market
Global Equestrian Helmets Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"




